PI Corner: Hot Topics
BBRAUN Pump Backflow Malfunction May Cause Underdosing or Overdosing of Medication
ISSUE: Backflow malfunction of the BBRAUN Infusomat Space Volumetric Infusion Pump System which may cause underdosing or overdosing of medication. Specifically, customers reported issues when attempting to infuse a secondary medication by connecting to the upper Y-site of the primary pump set (piggyback infusion). Rather than flowing towards the patient, secondary medication was observed backing up into the primary IV container.
MITIGATION: Corrective actions are being taking by BBRAUN now, however in the meantime, this is the recommendation: When administering secondary medications via piggyback please clamp the primary line above the upper Y site using the available slide clamp on the pump administration set. This will require unclamping of the primary line after delivery of the secondary medication.

Combined Use of the Military HAMILTON-T1 and Universal Portable Anesthesia Complete (UPAC) Vaporizer in the Pull-through Configuration May Cause Barotrauma Injury
ISSUE: The HAMILTON-T1 ventilator caused injury when connected to the UPAC vaporizer in a pull-through configuration with high-flow oxygen and FIO2 settings above 97%. The HAMILTON-T1 delivered uncontrolled high pressure followed by ventilator shutdown. This malfunction is reproducible when tested under identical conditions at Brooke Army Medical Center.
MITIGATION: Only use the UPAC Drawover in a push-through configuration when connected to a ventilator. This is reflected in the updated Universal Portable Anesthesia Complete (UPAC) Vaporizer And Mechanical Ventilation, Feb 2025 CPG which can be found on the JTS CPG page and in the Deployed Healthcare Resource Collection on the JTS Deployed Medicine website.
Instructions for the push-through configuration are available here.

Push-through configuration

Pull-through configuration
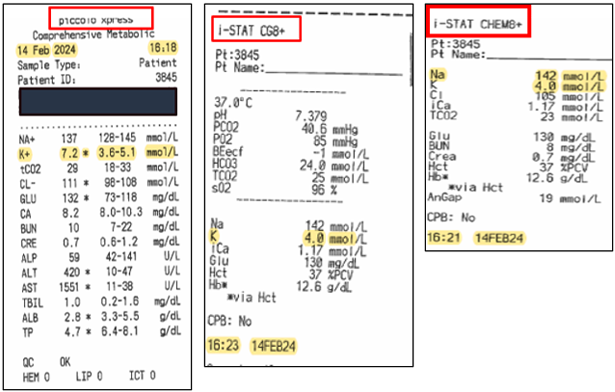
Piccolo Xpress Blood Chemistry Analyzer: False Readings
ISSUE: In cases of extreme muscle trauma or highly elevated levels of creatine kinase (CK), the Piccolo Xpress may report a falsely elevated potassium (K+) value.
RESOLUTION: When using the Piccolo Xpress in cases of extreme muscle trauma or highly elevated levels of creatine kinase (CK), unexpected high potassium (K+) readings need to be confirmed utilizing a different methodology, such as iSTAT.
EMV+ Zoll 731 Patient Disconnect Alarm
ISSUE: Four reported Zoll 731 Ventilator malfunctions from Jun 2020 to May 2021
PROBLEM: Patient Disconnect alarm prevents operation with NO break in circuit identified.
| Alarm Name | Mitigation/Resolution |
|---|---|
| Patient Disconnect | Description: Alarm triggers when the airway pressure fails to exceed the PEEP setting by ~ 7 cm H2O (hPa). When this occurs the user should check the patient connection, breathing circuit connections and the exhalation valve. At times this alarm can be caused by the patient breathing with the ventilator during inspiration which prevents the PIP from passing the minimum pressure. Mitigation/Resolution:1) Check patient connection, 2) Check circuit for loose hose/tube, 3) Check exhalation valve, 4) Check patient, and 5) Replace circuit If not resolved:Manually ventilate patient |
MITIGATION:
- Use only blue circuits for Zoll 731. (White circuits were for old Impact 754 ventilator.)
- Inspect for integrity of the circuits exhalation valve silicon diaphragm.
- Store blue circuits in environmentally controlled areas when possible.
- Use bacterial/viral filter to protect vent from dust. (apply to inlet valve on side of vent)
AFTER ANY TYPE OF VENT FAILURE, DO THIS:
- Inspect Zoll 731 inventory for current biomed safety certification (required every 6 months).
- Quarantine the Zoll 731 and circuit AS-IS for BIOMED.
- Submit the event to Joint Patient Safety Reporting.
- Work with BIOMED to complete a SF368 Product Quality Deficiency Report describing the malfunction or failure as well as repair
- Conduct frequent proficiency training and include in hand off training to replacement unit(s)

Use This Circuit

Circuit to Discard
*Use the white circuit for the Impact 754 ventilator only.
RESOURCES:
- Zoll Impact 731 Operating Guidelines Rev E, date: Mar 2020
- Initial and Sustainment Training:
Radio Frequency Identification Requirement (Oct 2021)
ISSUE: Retained Surgical Item (RSI) events continue to occur and put patients at risk. RSI is a "NEVER EVENT" which can happen due to urgency, distraction, time limitations, human error, miscommunication and other factors. Furthermore, surgical counts may be rushed or overlooked in emergency surgeries. A Root Cause Analysis conducted on a Dec 2019 "never event" prompted USCENTCOM leaders to direct units to employ Radio Frequency Identification (RFID) technology to help prevent RSI events.
RESOLUTION: The U.S. Central Command (USCENTCOM) Surgeon directed the procurement and use of RFID devices and solutions in all Role 2 and Role 3 USCENTCOM facilities by 01 Jul 2020 in a Jan 2020 USCENTCOM policy. A performance improvement project conducted in early 2021 resulted in the following policy revision: only Role 2-E (with air evacuation capabilities) and Role 3 units are required to have the RFID scanners. ONLY RFID sponges and towels will be used in procedures in the operating room (OR). OR teams must replace all custom surgical pack towels and sponges with RFID towels and sponges to prevent RSI events. Use of RFID technology also facilitates accurate surgical item counts. To learn more, refer to the USCENTCOM policy memo, Medical Policy for the Utilization of Radio Frequency Identification (RFID) Sponges/Towels, which was updated 09 Mar 2021. (CAC is required to access.)
REQUIREMENTS: Requirements include the RFID system, RFID supplies (sponges, towels), training, policy compliance, and local standard operating procedures. Teams can arrange RFID system training through unit leadership or the component CLINOPS Chief. Consult with your leadership if you do not meet the requirements.
Note: USMH-Kuwait, 411 HC was the first unit to complete RFID implementation and assisted with this update. Special thanks to MAJ Jeffrey Caliolio and 1LT Mayda Valle Valle for their contribution and video.
Ventilator Malfunctions (May/June 2019)
ISSUE: Uni-Vent/Eagle/Impact 754 mechanical ventilator equipment failures were reported to PI team via email and during JTCCCC discussions. The PI team investigated 13 incidents of ventilator malfunction from Apr 2017 to May 2019.
RESOLUTION: JTS led cross-agency communication and collaboration to address device failures. A safety alert, Medical Material Quality Control (MMQC) message, and clinician preflight checklist were issued describing steps to mitigate additional ventilator failures.
Preflight Mission Checklist and Routine End-User Preventative Maintenance Checks for Uni-Vent® Eagle™ 754
- Inspect hoses for cracking, discoloration and disfigurement; inspect tubing/fitting connections; replace defective parts.
- Inspect compressor inlet filter for dirt, lint or general wear and replace as needed.
NOTE: this filter must be replaced–not cleaned* - Confirm cyclical preventative maintenance and replacement of components; preventative maintenance is required after 6 months of non-use (non-use/storage alarm should initiate).
*Clinicians have used ventilator circuit filters when inlet filters are not available
Intraosseous (IO) Needle Size Complications (Nov 2019)
ISSUE: IO line malfunctions were suspected due to short needle length and/or mal-placement. Radiographic case review revealed IO needles failed to penetrate the bone cortex, preventing fluids and blood product administration.
RESOLUTION: JTS launched awareness campaign about the proper needle sizes, and discussed the correct needle specifications at continuing education conferences. End-user: select proper needle length; aspirate for bone marrow confirmation, flush with NS prior to use.
| Device | Sites | Pt Age/ Size | Needle Size |
|---|---|---|---|
| FAST Tactical | sternum | ›12 years | 1 size |
| EZ IO - pink | humerus, tibia | 3 – 39kg | 15mm, 15 G |
| EZ IO - blue | tibia; humerus if no excessive tissue | ›3kg size | 25mm, 15G |
| EZ IO - yellow | humerus | ›40kg, excessive tissue | 45mm, 15G |
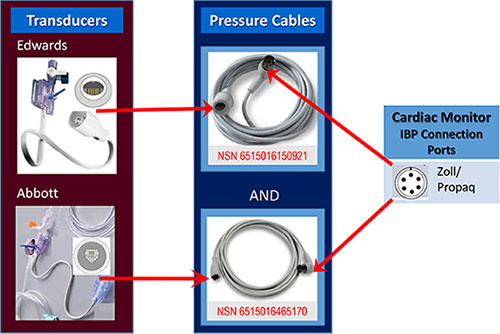
Invasive Blood Pressure (IBP) Cable Incompatibility (Jan/Feb 2020)
ISSUE: IBP transducers-pressure cables connection mismatch was identified in AOR. Problem prevented the ability to monitor intra-arterial pressures on a critically ill patient during transfer between roles of care. Investigation revealed long-standing inconsistency with IBP supplies.
RESOLUTION: JTS led a cross-agency working group to address supply inconsistencies. JTS helped inform AOR teams of the transducer-cable mismatch. A MMQC message was released providing awareness and National Stock Numbers (NSNs) so that AOR units may order and stock both types of cables. Capability managers were informed of updates needed to assemblages.
i-STAT Software/Device Lockout (Feb/Mar 2020)
ISSUE: An austere surgical team was unable to perform blood analyses during a trauma resuscitation. The i-STAT blood analyzer locked due to expired software. Investigation revealed lack of awareness of software update deadlines and that device PMCs were not synched with biannual manufacturer release of new software.
RESOLUTION: Analyzer software must be updated on time. Probe into the matter uncovered expired software. For future design, JTS conveyed to manufacturer impact of device lock-out for austere teams. JTS distributed software update information to clinicians advising proactive outreach by austere teams to MEDLOG. JTS also released just-in-time reminder to clinicians and Medical Logistics. For future design, JTS conveyed to manufacturer impact of device lock-out for austere teams.
| Software Release Date | Software Expiration Date (Suspense to upload new software) |
|---|---|
| ~27 Apr 2020 | *24 Jun 2020 |
| Nov 2020 | Dec 2020 |
| May 2021 | Jun 2021 |
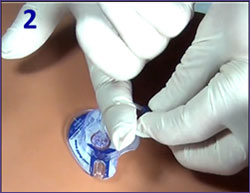
FAST-T Intraosseous (IO) – Problematic Removal (March 2020)
ISSUE: Reports of difficulty removing FAST-T IOs and at risk of tubing tip separation during removal. At least 1 IO tip retained in a patient's sternum.
RESOLUTION: JTS reviewed literature and found that IO removal and retained IO tip problems could likely be caused by improper removal technique. JTS distributed, to AOR clinical leaders, the key steps, instructions and manufacturer learning materials for proper EZ IO and FAST-T IO removal to prevent future problems. Information also presented at continuing education presentations.
Correct FAST-T Removal
- Detach infusion, remove dressing
- Critical: Bend FAST-T tubing over index finger to securely grasp tubing; trap leur connector with middle finger.
- Grip tubing firmly and as close to the patient's skin as possible.
- Do NOT hold down the target foot/ base.
May cause separation of metal tip.
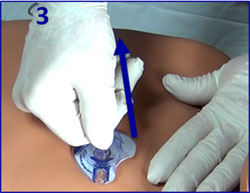
- Option 1 ("claw hammer"): Thumb holds tubing against index finger.
- In 1 swift, strong motion, rotate back onto little finger.
- Option 2 ("lawnmower pull"): Pull upward in steady, strong, consistent pull.
- Inspect that tubing tip was extracted intact.
EZ IO Removal
- Detach infusion, remove dressing
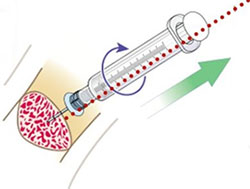
- Attach leur lock syringe
- Maintain axial alignment and rotate syringe clockwise while pulling straight back.
Do NOT rock the syringe! - Back catheter out while stabilizing the extremity
- Document IO malfunction and removal (whether line malfunctioned or not)
- Short progress note recommended
- Submit JPSR
Ready-Heat™ Hypothermia Prevention and Management Kit (HPMK, May 2020)
ISSUE: Skin burns were reported and suspected related to use of the Ready-Heat™, which is contained within the HPMK.
JTS is investigating reports of iatrogenic burns and possible variables to include: blanket time, product integrity, environmental/physiological conditions that may impact thermal effects. JTS is also in dialogue with product manufacturers. Clinicians are reminded to use a blanket or sheet barrier between patient and Ready-Heat™ and to report suspected patient injury by completing JPSR or notifying JTS: dha.jbsa.healthcare-ops.list.jts-pips@health.mil
On Exposed Skin

HPMK: Two-Part Kit
- HPMK®: Heat reflective shell
- Ready-Heat™ Blanket: Contents include iron powder, water, activated charcoal, wood powder, and salt